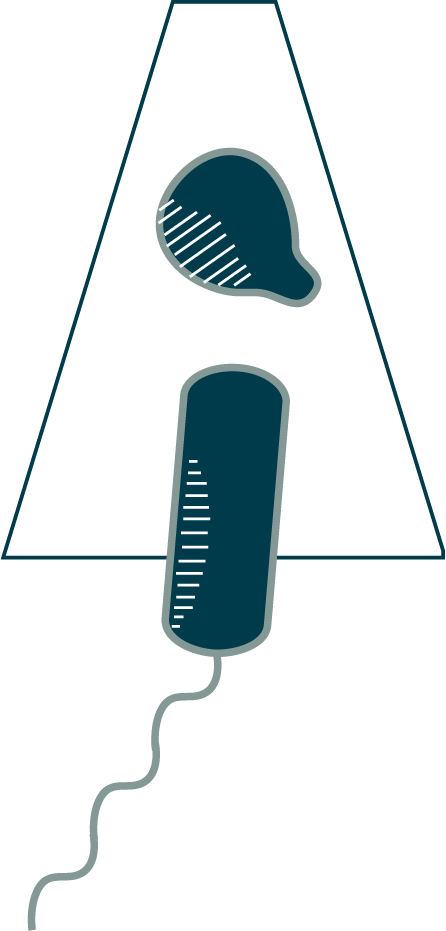
Once a Bdellovibrio bacteriovorus like this one has latched onto its unfortunate host, it opens a portal in the host’s outer membrane, secreting a cocktail of enzymes that alter the host cell wall, causing the cell to round up and enlarge the periplasmic space. The B. bacteriovorus quickly slides into the host periplasm through the portal (⇩) and seals the door behind it. In the process of preparing for entry, the cell dismantles equipment that is no longer necessary, including its chemosensory array and flagellum. As you can see here, the sheathed flagellum dislocates from the motor and is pulled into the periplasm, where the filament is degraded.
Note: The prey here–the small, round cell–comes from a mutant strain of Escherichia coli that has an altered form of the shape-regulating protein MreB, which results in thinner cells. It is also lacking the Min system that positions the division plane at midcell. As a result, division occurs randomly along the length of the cell. The cells that divide near the pole produce “minicells” like this one. Minicells do not contain a genome and are not viable cells, but they can be useful for experiments. In this case, the reduced thickness of the sample improves the quality of cryoET imaging. It also lets us capture intermediates in the B. bacteriovorus entry process.